The universe is built on small things. Atoms are so small you could line up a million of them across the width of a human hair.
Each atom is a very careful arrangement of protons, neutrons, and electrons. And most of the time, these parts work together in harmony, keeping matter stable and life possible.
But what if you changed that balance? Specifically, what if every atom in your body gained just one extra electron?
And yes, this question surely sounds like a line from a science fiction novel, but it’s an interesting way to explore the rules that govern our world.
By following the consequences of such a change, we can see why things work the way they do-and how fragile the order of life really is.
So, let’s see what would really happen in this imaginary hypothetical scenario.
Let’s start with some numbers.
Interesting fact: If you could count every atom in your body at one per second, it would take you over a billion times the age of the universe to finish.
Atoms and Numbers

Atoms are not just the building blocks of people-they make up everything we see and touch. To get a sense of scale, consider how many atoms are found in different familiar objects.
The numbers are almost beyond belief as you can see in the table below:
| Object | Approximate Mass | Estimated Number of Atoms |
| Human (70 kg) | 70 kg | 7×1027 |
| Dog (30 kg) | 30 kg | 3×1027 |
| Cat (4 kg) | 4 kg | 4×1026 |
| Chair (wood, 10 kg) | 10 kg | 6×1026 |
| Glass of water (250 mL) | 0.25 kg | 8×1024 |
The scale of the atomic world is almost impossible to picture, yet it’s what makes up everything we know.
For example, if we were to write out the smallest number here (8×1024) – it would look like this:
8,000,000,000,000,000,000,000,000!
That’s a number called septillion – so even ordinary glass of water consists of 8 septillion atoms.
Ok, so now that we know the scale we also briefly need to touch on the topic of the nature of atoms, before continuing to potential consequences of adding electrons to them.
The Nature of Atoms
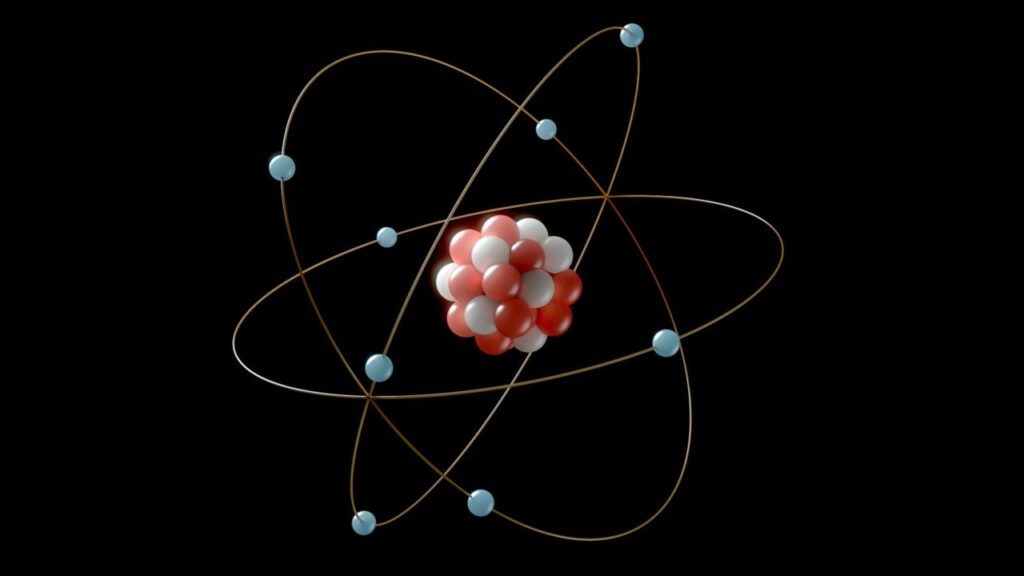
Atoms are the foundation of chemistry. And everything else for that matter. Each one has a dense core, the nucleus, made of protons and neutrons. Protons carry a positive charge, while neutrons are neutral.
Electrons, which are much lighter, orbit the nucleus and carry a negative charge. The number of protons defines the element-hydrogen, carbon, oxygen, and so on.
The number of electrons usually matches the number of protons, making the atom neutral.
And so, the balance between positive and negative charges is not just a technical detail. It is what allows atoms to form molecules, and molecules to form the tissues and organs of living things.
The way electrons are arranged around the nucleus determines how atoms stick together, how they react, and how they build the complex chemistry of life.
Interesting fact: The diameter of a typical atom is about one ten-millionth of a millimeter.
What Happens When You Add an Electron?

When an atom gains an extra electron, it becomes a negative ion. This might seem minor, but it changes everything about how the atom behaves.
And in the body, ions are very important. Sodium, potassium, and calcium ions help nerves send signals and muscles contract. But they only do that if they are in a complete balance.
If every atom in your body gained an extra electron, every single one would become a negative ion. The result would be a body made entirely of atoms that repel each other.
The chemical bonds that hold molecules together depend on the sharing or transfer of electrons in very specific ways. With every atom overloaded, those bonds would break.
The body’s structure would disentangle at the most basic level.
Interesting fact: The word “ion” comes from the Greek word for “going,” because ions move when placed in an electric field.
The Charge Imbalance

Let’s try to picture the scale of this charge. The human body contains about 7 octillion atoms. Each electron carries a tiny negative charge, but together, they add up.
If you added one electron to every atom, your body would suddenly have a negative charge of billions of coulombs (standard unit of electric charge). For comparison, a lightning bolt carries about 3 coulombs.
This charge would not stay put.
The atoms, now all negatively charged, would push away from each other with incredible force. The repulsion would be so strong that the body would not just fall apart-it would be torn apart at the atomic level.
The energy stored in this charge imbalance would be released in a brutal burst.
Interesting fact: The force between two electrons is about 10^39 times stronger than the force of gravity between them.
The Explosion

The sudden release of energy would be catastrophic. The body would not simply break into pieces; it would vaporize.
The atoms would be ripped from their bonds, and the energy released would create a blast far beyond any chemical explosion. The heat and shockwave would destroy anything nearby.
And btw, this would not be a slow process. It would happen in a fraction of a second.
The body would become a cloud of plasma-a mix of charged particles moving at high speed. The explosion would be more like the detonation of a small nuclear device than anything we see in ordinary life.
Interesting fact: Plasma, the state your body would become, makes up over 99% of the visible universe, including stars.
The End of Biochemistry
Life is chemistry in motion. It’s that simple. Enzymes, DNA, proteins, and cell membranes all rely on precise arrangements of atoms. The bonds that hold these molecules together are rather sensitive to charge.
Add an electron to every atom, and these bonds break apart. The molecules that make life possible would disintegrate.
Cells would lose their structure. The instructions for life, written in the code of DNA, would vanish as the molecule fell apart.
No process in the body could continue. The machinery of life would go to a complete halt in an instant.
Interesting fact: DNA is so stable in its usual form that it can last for thousands of years in the right conditions.
Collateral Damage
The effects would not stop with the person. The sudden release of energy and charge would affect the surroundings. The shockwave could break windows, knock down walls, and harm anyone nearby.
The charge would try to escape, possibly creating a massive arc of electricity to the ground, like a supercharged lightning bolt.
The ground itself might also be scorched, and electronics in the area could be fried by the electromagnetic pulse.
One thing is certain – this event would be remembered as a complete and utter disaster.
Interesting fact: The electromagnetic pulse from a nuclear explosion can knock out power grids over hundreds of miles.
What About Humanity as a Whole?

Now, stretch your imagination. If this happened to every person on Earth at once, cities would vanish in bursts of light. The atmosphere would be filled with plasma clouds.
The sudden release of charge could disrupt the planet’s magnetic field, and the surface could be scarred by lightning-like discharges.
Or, picture a single person in a crowd suddenly turning into a ball of plasma. The event would be so fast and violent that it would leave behind only a burned mark and a memory.
Interesting fact: Static electricity from walking on a carpet can reach thousands of volts, but the current is tiny, so it’s usually harmless.
What About the Universe?
As mentioned at the beginning, atoms are not just the building blocks of people-they are the building blocks of everything. The same rules that keep your body together also hold together stars, planets, and galaxies.
The balance of charge is a rule that applies everywhere.
If you could add an electron to every atom in a star, the result would be just as dramatic. The star would break apart, releasing its energy in a burst.
Namely, the universe depends on the careful balance of charge, and even a small change can have dramatic effects.
Interesting fact: The sun loses about 4 million tons of mass every second as energy, but its atoms remain balanced in charge.
Could This Ever Happen?

Adding an electron to every atom in a person is not just a technical challenge-it’s a physical impossibility with our current understanding of science. The forces required to push an extra electron into every atom in your body are enormous. Each atom naturally resists this change, as its electrons are already arranged in stable shells.
Forcing an extra electron onto every atom would demand energy on a scale far beyond anything we can produce, even in the most advanced laboratories.
No technology, not even the most powerful particle accelerators, can achieve this for more than a few atoms at a time.
In fact, when scientists add or remove electrons in experiments, they do so with single atoms or small molecules, never with entire objects, let alone something as complex as a living body.
Even if it were possible to add all those electrons, the result would be instant and catastrophic. The body would not have time to adjust or adapt.
The sudden imbalance would overwhelm the natural forces that hold atoms and molecules together, leading to immediate and violent disintegration.
So, to answer the question directly-NO, this is not possible in any practical sense. The laws of physics and chemistry keep matter stable and life possible by making such scenarios impossible.
For now at least 😊
Interesting fact: The Large Hadron Collider smashes particles together at nearly the speed of light, but it cannot change the charge of every atom in a person.